1/ Responses of human respiratory epithelium to particle-induced environmental stress (Armelle Baeza (group manager), Sonja Boland, Stéphanie Devineau, Francelyne Marano (emeritus)).
Human activity (industry, transport) and especially the use of nanotechnologies results in an increase in the exposure of populations to atmospheric pollutants as well as to engineered nanomaterials. The epithelial cells lining the respiratory tract are the first target of inhaled particles, and develop specific responses to this type of exposure.
Our research aims to relate the biological responses with certain physico-chemical characteristics of the particles (chemical composition, size, specific surface, surface reactivity, crystallinity, shape, oxidative potential…). We study engineered nanoparticles as well as nanoparticles unintentionally produced by combustion (diesel engine, chimney fires) or by wear processes (brake pad).
We attempt to improve understanding of the cellular and molecular mechanisms induced by these particles and especially their ability to generate an oxidative stress related or not to their intrinsic oxidative properties.
![<multi> [fr] Particules ultrafines et nanoparticules manufacturées [en] Ultrafine particles and engineered nanoparticles </multi>](https://bfa.u-paris.fr/equipe-6/wp-content/uploads/sites/7/2017/01/png_Fig1_NP_EN.png)
![<multi> [fr] Particules et stress oxydant [en] Particles and oxidative stress </multi>](https://bfa.u-paris.fr/equipe-6/wp-content/uploads/sites/7/2017/01/png_Fig2_ROS_mechanism_EN-3.png)
Oxidative stress can activate signalling cascades leading to adaptive responses or cell death. We also study the nucleolus, a nuclear domain dedicated to ribosome biogenesis and considered as a sensor of cellular stress.
We favor in vitro approaches to investigate the direct effect of the particles on respiratory epithelial cells by using cell lines as well as primary cultures of the human bronchial epithelium. The latter are able to differentiate in vitro and to develop a mucociliary epithelium that can be preserved in culture for several months allowing performing repeated exposures at low doses and studying long term effects.
![<multi> [fr] Travaux de l’équipe [en] Team research </multi>](https://bfa.u-paris.fr/equipe-6/wp-content/uploads/sites/7/2017/01/png_Fig3_Team_research_EN.png)
In particular, the team focuses on:
- internalisation mechanisms and those of passage of particles through airway epithelial barrier
- adaptive responses (such as the anti-oxidant and pro-inflammatory responses) triggered by particles and the cell signalling pathways involved
- the effect of particles on the repair and protection functions of the epithelial barrier and their impact on the differentiation of the respiratory epithelium using omics approaches especially on the epithelial secretome.
- the modulation of these different effects by the interaction of particles not only with biomolecules (lipids, proteins) forming a corona around particles but also with xenobiotics.
Our researches aim to contribute to establish adverse outcome pathways through identifying mechanisms of toxicity and developing 3D culture models.
2/ Cellular and nucleolar stress (Pascal Roussel, Valentina Sirri-Roussel).
We study the role of the nucleolar NAD+-dependent deacetylase sirtuin 7 (SIRT7) in the synthesis of ribosomal subunits. We revealed that SIRT7 activity is a critical regulator of processing of 45S, 32S and 30S pre-rRNAs.
We demonstrated that naphthoquinones menadione and plumbagin inhibit SIRT7 in vitro and in vivo. We also established that sulfhydryl arylation by menadione or plumbagin could be prevented by the thiol reducing agent N-acetyl-L-cysteine, with concomitant blockade of inhibition of SIRT7 catalytic activity. This inhibition of SIRT7 could be crucial in defining menadione or plumbagin as anti-tumor agents.
3/ Enzymes, environment, molecular pathology (Linh-Chi Bui, Florent Busi, Frédérique Deshayes, Jean-Marie Dupret (group manager), Emile Petit, Justine Renault, Fernando Rodrigues-Lima (group manager), Mireille Viguier).
We seek to characterize at the molecular and functional levels the interactions between xenobiotics and enzymatic pathways.
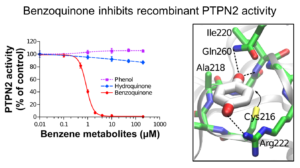
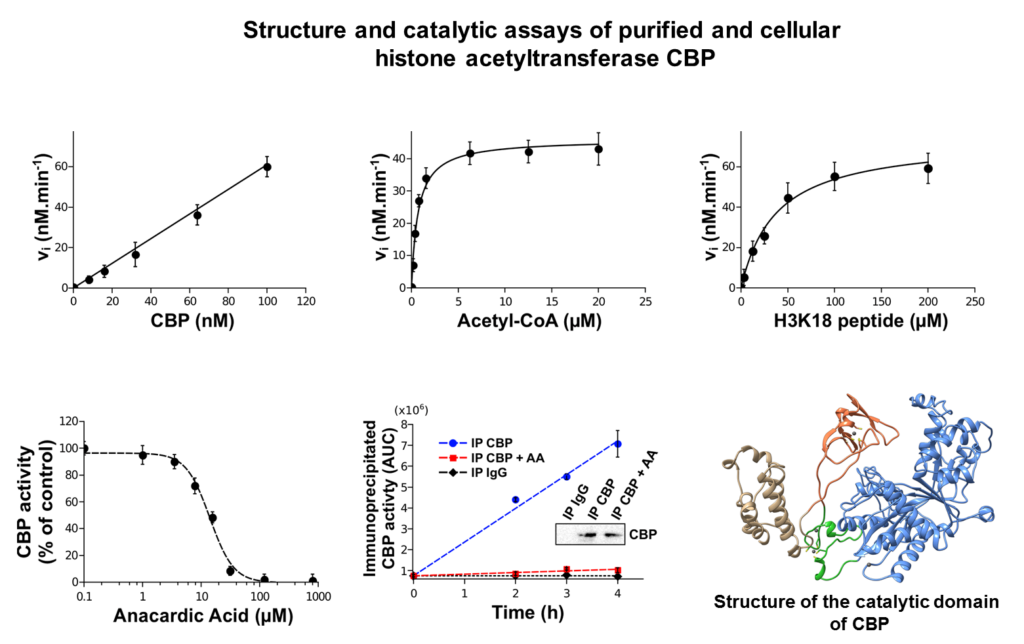
We focus mainly our work on the impact of leukemogenic chemicals (benzene, etoposide,…) on the tyrosine phosphatase PTPN2, the histone acetyltransferase CREBBP and the histone methyltransferase SETD2.
We also study the structural, molecular and functional impacts of pathogenic mutations on these enzymes in the context of malignancies.
These three enzymes (PTPN2, CREBBP and SETD2) play key roles in cell signaling and/or epigenetic processes and have been shown to be involved in normal and malignant hematopoiesis. We demonstrated through different molecular and cellular approaches that leukemogenic quinone metabolites irreversibly impair the functions of these enzymes through covalent modification of critical catalytic or structural cysteines with subsequent impact on cell signaling and on epigenetic modification of histones. In parallel, we carried out structural and enzymatic approaches that allowed us to characterize the first crystal structure of a pathogenic mutant of PTPN2 (C216G) and the functional impact of this mutation.