1) Aging: fundamental mechanisms, interactions with environment and search for anti-aging compounds
Aging is a process that remains poorly understood despite significant progresses over the last three decades. Moreover, its deleterious consequences, in particular the increasing incidence of age-related diseases, have a strong impact at the socio-economic level, given the demographic evolution of human societies. It is therefore critical to progress in our understanding of fundamental mechanisms involved in aging and its associated pathologies.
For this purpose, we are developing genetic and functional genomic approaches in Drosophila. We have identified gene networks involved in cardiac aging. We are also interested in the impact of environmental factors modulating metabolism and longevity. We have recently observed environmental interventions confering increased longevity over several generations. We are currently investigating the mechanisms involved in this memory effect.
In parallel, a collaboration with the Chinese company Infinitus has enabled us to identify compounds from traditional Chinese medicine with the capacity to counteract processes related to aging (resistance to stress, cardiac dysfunction, reduction of locomotor activity, etc.) and to confer protection in a neurodegenerative context.
2) Neurodegenerative and cardiac disease
We use Drosophila to modelize degenerative diseases, in order to explore physiopathological mechanisms and to test therapeutic strategies. Our approaches rely on the genetic and pharmacological screening capacities of this powerful model organism.
2-a. Polyglutamine diseases (Huntington’s disease (HD) and Spinocerebellar ataxia type 3 (SCA3))
These two diseases are due to expansions of CAG triplets, encoding glutamine, in the coding regions of the Huntingtin and ataxin 3 genes, respectively. Both are proteinopathies, characterized by the appearance of intranuclear aggregates and degeneration of specific regions of the central nervous system such as the cerebellum, pons, cortex,…
In the framework of the European program TreatPolyQ, we collaborated with the teams of L. Pereira de Almeida (Coimbra, Portugal) and T. Schmidt (Tübingen, Germany) to study the role of some microRNAs and nuclear export factors in SCA3 pathology. Using the Drosophila model, we identified a nuclear transporter of the karyopherin family, KPNA3 (also called Importin subunit alpha-4) as a key element in the nuclear localization of ataxin 3. Thus, the decreased KPNA3 expression relocates the mutant SCA3 protein in the cytoplasm of cells and ameliorates the degeneration observed in the Drosophila eye in pathological context, making KPNA3 a potential therapeutic target of interest.
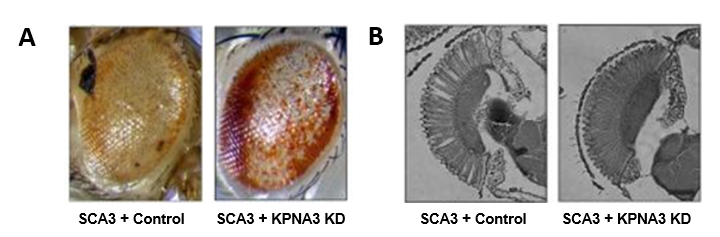 Figure 1 : A. In control conditions (SCA3 + Control), the expression of pathological ataxin 3 in the Drosophila eye leads to morphological defects: depigmentation, necrosis, general disorganization (left image), that the decrease in KPNA3 expression (SCA3 + KPNA3 KD) allows to improve (right image): partially repigmented and partially reorganized eye. B. Observation of the retina of these flies shows a clear improvement of the photoreceptor morphology when KPNA3 expression is decreased (image on the right) compared to control conditions, for which vacuoles and photoreceptor disorganization are observed in the retina.
Figure 1 : A. In control conditions (SCA3 + Control), the expression of pathological ataxin 3 in the Drosophila eye leads to morphological defects: depigmentation, necrosis, general disorganization (left image), that the decrease in KPNA3 expression (SCA3 + KPNA3 KD) allows to improve (right image): partially repigmented and partially reorganized eye. B. Observation of the retina of these flies shows a clear improvement of the photoreceptor morphology when KPNA3 expression is decreased (image on the right) compared to control conditions, for which vacuoles and photoreceptor disorganization are observed in the retina.
We have also developed a cardiac model of Huntington’s disease, and more recently an inducible glial model. The latter is complementary to existing neuronal models and allows us to explore the respective consequences of pathological Huntingtin expression in different cell types of the central nervous system (glia or neurons) on various physiological parameters (longevity, locomotor activity, circadian rhythm ..). We have thus identified strong differences between neuronal and glial models of HD, which illustrates the complexity of the disease. We performed a genetic screen to identify the involved signaling pathways and, on this basis, to explore new therapeutic strategies. We have shown that partial depletion of the transcription factor nej/dCBP in glial cells is protective, restores locomotor activity in HD flies and increases their lifespan. Thus, dCBP contributes to the development of the disease in glial cells, contrary to what was observed in neurons. Our data therefore suggest that combinatorial approaches with tissue-specific targeting may be necessary to discover effective therapies against HD.
New Drosophila models of HD, developed using CRISPR/Cas9 techniques, are also currently studied.
2-b. Freidreich’s Ataxia (FA)
FA is a mitochondrial disease affecting the nervous system and the heart. It is due to a GAA expansion in the first intron of the FXN gene leading to decreased expression. FXN encodes frataxin, a small mitochondrial protein involved in the synthesis of iron-sulfur clusters and highly conserved during evolution.
We first established a cardiac model of FA in Drosophila and showed that the cardiac-specific inactivation of frataxin leads to cardiac dilation with loss of cardiomyocyte contractility and alteration of sarcomeres. We have performed pharmacological screens on this cardiac model and identified several molecules with cardioprotective effects including methylene blue.
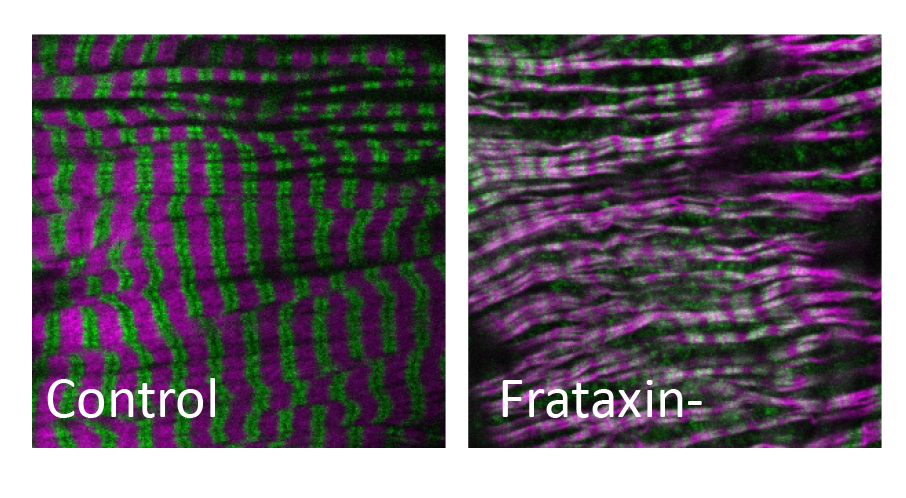 Figure 2 : Alteration of sarcomeres (Actin in magenta and Myosin in green) in cardiomyocytes of flies in which frataxin has been inactivated
Figure 2 : Alteration of sarcomeres (Actin in magenta and Myosin in green) in cardiomyocytes of flies in which frataxin has been inactivated
Our current approaches are based on new models of FA obtained by humanization of the Drosophila frataxin gene. For this purpose, we have inserted into the intron of the Drosophila frataxin gene, intronic sequences of the human gene carrying GAA expansions of various sizes. These expansions lead to a decrease in the expression of the gene and reproduce symptoms associated with the human disease (locomotor and cardiac deficits, reduced viability). We are currently using these new models to develop pharmacological approaches (secondary screening of chemical libraries and evaluation of therapeutic peptides). Our studies rely on national and international collaborations and are supported by the French association AFAF (Association française de l’Ataxie de Friedreich) and the American association FARA (Friedreich Ataxia Research Alliance).
2-c. Parkinson’s Disease
Parkinson’s disease (PD) is the second most common neurodegenerative disease after Alzheimer’s disease. It is estimated to affect 1% of people over 65 years of age. It is characterized by slow and progressive loss of dopamine neurons located in the substantia nigra pars compacta of the brain, which leads to abnormal movements but also to cognitive disorders. There is currently no curative treatment for this disease, which makes it a real public health issue. In this context, we are using Drosophila models of PD to identify mechanisms involved in the pathogenesis of the disease that can be used as therapeutic targets. Recently, the LRRK2 gene, which is involved in both sporadic and familial forms of PD, has been identified as a key therapeutic target. More specifically, the phosphorylation state of LRRK2 seems to influence its physiological and pathological function. We have therefore developed national collaborations to identify and validate phosphoregulators of LRRK2 that can modulate its phosphorylation state and therefore could be potential drug candidates.
This collaborative work was initiated within the framework of an « Emergence in Research » funding (Idex University of Paris) and is now supported by the ANR (PARK-PEP project).
2-d. Screening in flies of variants identified in human patients with cardiomyopathies
Drosophila has emerged in recent years as a relevant model to identify genes involved in the development of cardiomyopathies and to study the mechanisms involved in their pathogenicity. Thanks to an in vivo cardiac imaging system developed in the team, we use this organism to study genes for which variants have been identified in patients by genomic approaches performed by our collaborators. This approach allows an in vivo functional screening of candidate genes in order to quickly provide arguments in favor or not of their pathogenicity.
 Figure 3 : In vivo cardiac imaging in Drosophila.
Figure 3 : In vivo cardiac imaging in Drosophila.
Anesthetized Drosophila expressing a GFP fluorescent protein in the heart are placed on a slide under a stereomicroscope. The emitted fluorescence light is captured through the dorsal cuticle by a high-speed camera, allowing the extraction of cardiac parameters.
3) Search for efficacy biomarkers for monitoring patients with neurodegenerative diseases: application in case of treatments
Deregulation of the metabolism of monocarbons is associated with an increased incidence of several chronic diseases, such as hyperhomocysteinemia, Alzheimer’s disease and other metabolic diseases such as type 2 diabetes and obesity. Although the molecular mechanisms that trigger Alzheimer’s disease are not fully understood, recent data suggests that dyslipidemia may contribute to its progression. Some patients with Down’s syndrome develop dementia comparable to that of Alzheimer’s disease at the age of 30-40 years. Pathological aging in the subject with trisomy 21 is associated with dementia syndrome which combines, to varying degrees, disorders of cognitive functions and behavior modifying the personality. These patients also have dyslipidemia with deregulations of the metabolism of monocarbons. Among the genes located on chromosome 21, the expression of DYRK1A, a serine threonine kinase, and CBS (cystathionine beta synthase), an enzyme involved in the metabolism of monocarbons, is deregulated in Alzheimer’s disease. We have demonstrated a relationship between DYRK1A and CBS and their involvement in the metabolism of monocarbons, cholesterol and insulin, all metabolisms deregulated in Alzheimer’s disease, hyperhomocysteinemia, trisomy 21 and type 2 diabetes. Targeting these two proteins for pharmacological treatment requires a better understanding of their function and of their interacting partners. The search for interactants, in collaboration with Dr JM Camadro (Jacques Monod Institute, UMR CNRS 7592), has enabled the identification of proteins already shown as potential biomarkers in the serum of patients suffering from Alzheimer’s disease. These different biomarkers are currently being analyzed in plasma and cerebrospinal fluid from patients with Alzheimer’s disease and patients with trisomy 21 with or without dementia and at the prodromal stage (in collaboration with Dr Anne-Sophie Rebillat (Institute Jérôme Lejeune, Paris) and Pr J Fortea (Memory Unit, Department of Neurology, Hospital de la Santa Creu i Sant Pau-Biomedical Research Institute Sant Pau-Universitat Autònoma de Barcelona, Barcelona, Spain).The evaluation of these biomarkers in the patient’s serum combined with imaging parameters and cerebrospinal fluid markers should make it possible to develop a prognostic / diagnostic test with the aim of identifying people at high risk of developing Alzheimer’s disease and who will be able to benefit from treatments.
In addition to validating new blood biomarkers, brain-plasma evaluation in mouse and rat models allows us to study the neurobiological mechanisms underlying deregulation of these biomarkers. To this end, we seek to demonstrate their role in the progression of Alzheimer’s disease by using the first inducible and progressive rat model of the disease, in collaboration with Dr Jérôme Braudeau (AgenT).
These mouse models also allow us to search for treatments targeting DYRK1A and CBS using a computational approach and in vitro and in vivo tests (as part of a consortium, Fig 3).
Figure 4: Consortium for the research of inhibitors by computational approach and in vitro and in vivo tests
As part of the research for treatments, these biomarkers are also used at the preclinical stage in order to analyze the effects of treatments at an early stage, which we are currently developing in two main directions:
1/a way targeting more specifically the activity of DYRK1A with a specific pharmacological inhibitor (in collaboration with Dr Yann Hérault (Institute of Molecular and Cellular Biology, UMR 7104 of CNRS, U1258 INSERM, Strasbourg) and Dr Laurent Meijer (Perha Pharmaceuticals, Roscoff)).
2/ a way to test a molecule at the embryonic stage (in collaboration with Pr François Vialard (Université Paris-Saclay, UVSQ, INRAE, ENVA, BREED, 78350, Jouy-en-Josas, France) and Pr Anne-Claude Camproux (Université de Paris, BFA, UMR 8251, CNRS, ERL U1133, Inserm)).
As part of a clinical study, a first phase II randomized trial with placebo, which was conducted by different teams including our group, made it possible to demonstrate that EGCG improves cognitive and learning capacities. young adults with Down’s syndrome (Fig. 4). This study is currently being continued in young children with Down’s syndrome, after determining the dose and efficacy using a preclinical model, as part of a phase II study in collaboration with Dr Cécile Cieuta -Walti (Jérôme Lejeune Institute, Paris) and Pr Rafael de la Torre (IMIM-Hospital del Mar Medical Research Institute, Barcelona).
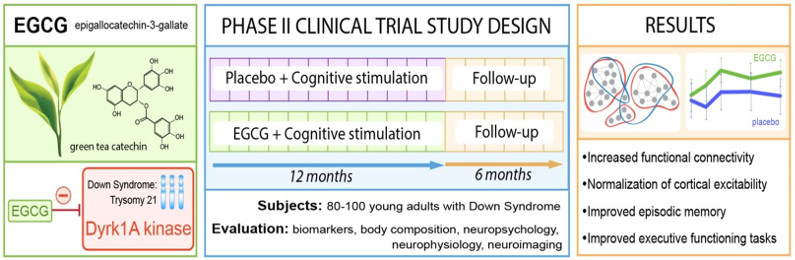
Figure 5: Principle of the phase 2 clinical trial with EGCG, a DYRK1A inhibitor, in patients with Down’s syndrome